OnRule publications - Series 2
Publications
Special topic for Product Regulatory Compliance
Compliance Model Number and Product Tree
By Tom Killam
July 26, 2022 | Series 2 / No. 2 | V1.0
The Compliance Model Number (CMN) is the subject of most Product Regulatory Compliance test reports, certifications, and self-declaration records as it relates to the product numbering. Yet, few companies use this notion to their advantage. Those that are using CMN strategically are saving significant amount of time and resources in getting their products certified, becoming more competitive in the global marketplace. Some companies term it as a “Regulatory Model Number” (RMN).
This Paper illustrates common issues with model naming we have encountered over years of product compliance experience, and describes best practices used by leading organizations to define cost-effective CMN and product tree structure.
This Paper is intended to benefit the Compliance, Product Management, Engineering and Marketing leaders at manufacturing companies with products that require regulatory compliance and Homologation in areas such as Safety, EMC, Radio, Telecommunications, etc. Product Regulatory Compliance Consultants, Agents, Test & Certification Laboratories, and Regulatory Agencies can also benefit from reviewing and implementing the recommendations in this paper.
The Problem:
A company defines a unique model ID for each configuration of a product. After going through the processes of testing, certification and, in many cases, global homologation, for all these configurations, the company obtains proof of compliance in the form of documentation that refers to the above-mentioned model IDs. Typically, there is some fixed cost associated with homologating each model number of a particular product. Using the model number for each configuration strategy will result in sums of money being collected for each model number identified times the total number of model numbers being added and tracked in the regulatory approvals documentation. If the compliance documentation simply referred to a single Compliance Model Number the extra costs for each additional model number would no longer be necessary.
Additionally, more issues arise when…
- The Company introduces yet another configuration of the same product with new model ID or
- For whatever reason, the company wishes to change the model ID for a particular configuration (e.g., change in color of a model).
In both these cases, the company needs both proof of conformity documentation to reflect the new or updated model IDs, and all the regulatory authorities that have blessed the previous models of the product need to add the new model designation to their list of approved products. Even if retesting is not required, in order to add the new or updated model ID to an existing set of documents, the company needs to work with the appropriate entities to reissue each of these documents, a process that is neither free nor fast. After any necessary testing has been completed (if required), then the company must contact each of the regulatory approval agencies that have previously approved the product and provide information about the new model number and pay to have the records updated to add the new model number. Additionally, the product marketing and sales teams will need to develop and provide documentation for each of the new models which is very time consuming and inconvenient.
The Solution:
The basic principle behind developing a model naming strategy for compliance purposes is relatively simple. The compliance model name used in regulatory documents should be a common model name to all of the configurations that utilize a common fixed platform (i.e., common motherboard, common enclosure or common power supply and cooling, one umbrella product identification that covers all possible product configurations). However, the implementation requires close collaboration among all parties identified in the beginning of this paper.
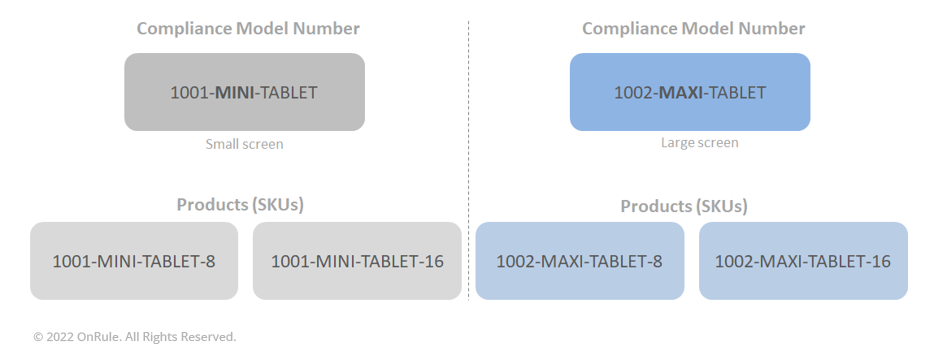
In the above simplified example, two tablets, one with a small screen and another with a large screen offer two configurations each. Both the small screen and large screen tablets are available with 8 GB storage or 16 GB storage. Therefore, we are proposing two Regulatory Model Numbers (RMN), one each for a small screen and large screen. Further, we are proposing two Products (SKUs) reporting to each of the Compliance Model. In our scenario, the OEM will test the Compliance Model only at the maximum configuration.
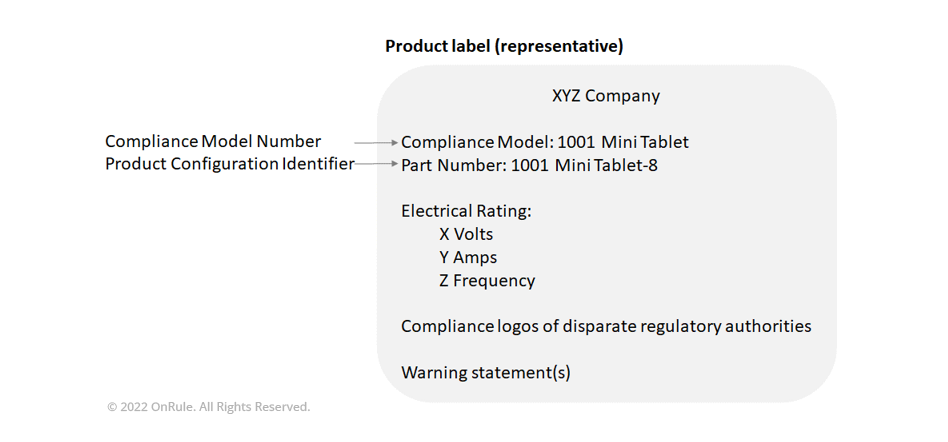
For a start, companies should develop a product hierarchy that will utilize a common CMN for the various configurations available for the product. Under this CMN, the various permutations of the product should be identified by some additional nomenclature other than model number. This can be accomplished by including both the CMN and the product configuration identifier (Part Number, Configuration Number, Service Tag Number or any other way to identify the particular configuration) on the compliance label. Necessitating a level in the product tree that is owned by the Product Management and Marketing teams, CMN underscores the importance of product regulatory compliance in the product lifecycle! The Compliance team must play a leadership role in defining the CMN, and must collaborate closely with the Product Management and Engineering team to integrate it in the product tree.
For any new product, the Engineering and Compliance groups together with their testing and certification partners should identify, develop, and execute test plans that account for all possible product configurations.
It is not necessary for all the possible configurations to fit into a single system for testing purposes. Many times, the list of possible configurations will require more than one system to represent all the hardware that will be necessary for complete test coverage. However, this is OK, and one can assemble as many systems as necessary for coverage of all hardware. Each system will need to be tested to ensure that all the hardware results in systems that will comply with the necessary requirements. Note that all configurations are still covered under one CMN.
Once all the testing has been completed successfully, it is important that the test reports reflect the alternate configurations that are available for the CMN assigned to this product group.
Anticipating future changes and testing against all possible configuration permutations requires more work and is more costly than simply testing and certifying a single configuration, but this approach has cost advantages in the long run. When testing and certifying multiple configurations in the same project, the cost can increase the project invoice by 15-20%, the cost of retesting and reissuing documents can double the overall certification costs and significantly delay new product launches. Additionally, the documentation required to provide to the distribution channel partners and sales team also increases, adding to the overall complexity. Therefore, the benefits clearly outweigh the costs.
Finally, once all the necessary configurations have been tested and certified, the product is to be marked as the Compliance Model identical to the model identified in the compliance documents. Each individual product unit will display in its compliance label two pieces of information: first the Compliance Model ID as listed in the compliance documentation, and second the Configuration ID to identify the product configuration. Note that the latter has no value for regulatory authorities and customs agencies, but informs the distribution partners, service chain, and customers of what is the exact configuration in a specific unit. In our previous example of a tablet, the Compliance Model ID would be 1001-MINI-TABLET and the Configuration ID would either be 1001-MINI-TABLET-8 or 1001-MINI-TABLET-16 (depending on the size of the storage or memory used).
It is extremely important to ensure that all parties in the manufacturing and distribution chain are trained on the concept of Compliance Model Number and configuration part number. It is important to ensure that shipping documentation have invoices and other technical papers with the correct CMN and not the particular configuration number listed or there will be issues with clearing customs and other shipping logistics.
Some companies try to mitigate model naming problems by issuing self-declarations of similarity (Declaration of Similarity or DoS), claiming the new model ID is sufficiently similar to another already certified model ID to avoid reissuing the documentation. This approach does not meet regulatory and customs requirements, and even though this is sometimes sufficient to demonstrate conformity in some markets, in most cases the risk of “stop ship” is high.
Another approach we have seen is using wildcards in the model ID definition. For example, the company and conformity documentation specify the model ID as ABC-1XXX where XXX can be any number from 000 to 999. This way, the theory goes, a product with the model ID label ABC-1234 will be covered under the umbrella conformity documentation. In practice, however, this solution is explicitly not valid in some countries.
Compliance testing requirements after a product has been certified:
As changes in the original configuration are developed, it will be necessary to perform some sort of maintenance on the regulatory files to address the changes and any concerns about the new components’ effect on the regulatory compliance.
For the discipline of Product Safety, this is usually accomplished by having multiple sources listed for the critical components used in the system. These lists of possible components to be assembled into the system should include all the safety critical approved or separately evaluated safety critical components used. The list should include components utilized in any of the system configurations that are to be covered by the report. In general, because of the constant changes in the electronics industry, it is a good idea that the list of components includes generic alternates providing some allowances to deal with component obsolescence and new technologies that would not adversely affect the Regulatory Compliance status. This is typically allowed for Safety Extra Low Voltage (SELV) plug in cards and Storage Devices.
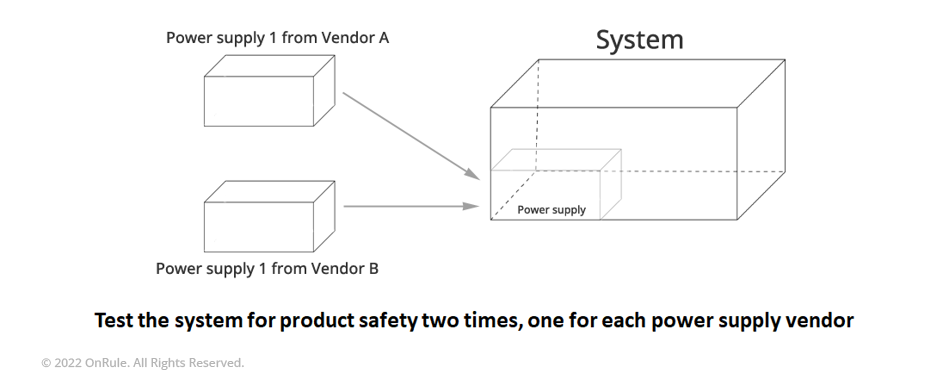
This is usually accomplished by adding separate lines in the list of critical components that state that additional types of a particular component are acceptable as long as the type of component is not changing, and the new component does not draw more power than its predecessor. This has been accomplished by listing the voltages and currents that are available for the component’s consumption in the critical components table on the line associated with the generic alternate together with the necessary regulatory approvals needed for the particular component.
If the changes cannot be addressed solely by adding to the Critical Components List and some retesting is necessary, then it will be required to have a revision to the Compliance Safety report that would include all necessary testing and associated updates.
For the discipline of EMC, certain types of alternate components will have significant impact on the compliance for the system, such as Power Supplies and new I/O ports. These types of alternate components will probably require complete retest for compliance with emissions and immunity requirements. In general concerns over adding other non-immunity effecting types of alternate components to a system are typically centered around the radiated emissions compliance from the product.
For these types of alternate components that were not tested in the original EMC system configuration, continued compliance will require some sort of limited internal evaluation to determine that their introduction will not have a negative effect on the EMC compliance for the system. Many times, this can be accomplished by performing an A/B Test (testing conducted to compare the performance of two versions of a single variable, in our example – three Hard Disk Drives from three different capacities), to show that the changes will not cause EMC to fall out of compliance.
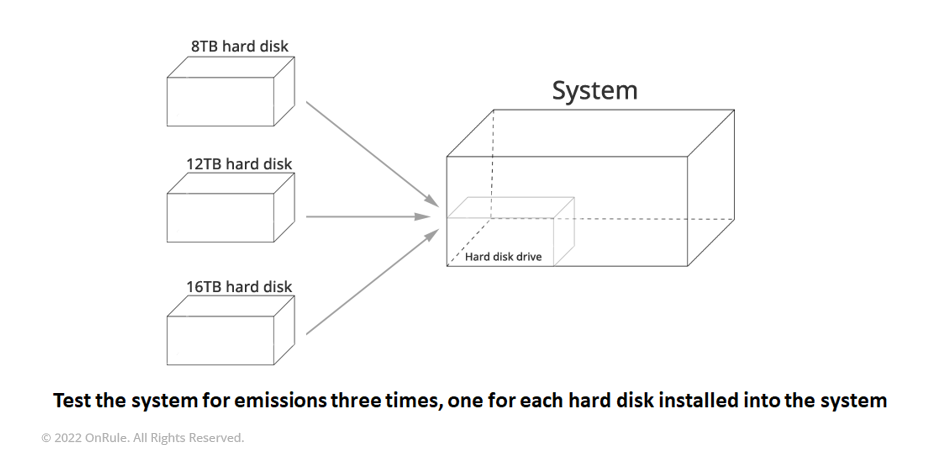
This can be accomplished by creating a test system that is exactly the same configuration as the system that was originally configured using all the components that were originally present. This system is then subjected to an EMC radiated emission scan to set a baseline for the emissions from the system. Following the test, the system is reconfigured to change the original component to the alternate candidate that is being evaluated. The system is then retested in the same environment using the same criteria to determine if the changes to the system have made any significant changes to the baseline that was created in step one. If the results are comparable, then the alternate component should be added to the Bill of Materials and the Engineering Test report that was created with the A/B Test should be kept on file should any questions arise about the alternate component’s influence on system compliance.
Even though it may be necessary to perform some file maintenance or report updates after all the new testing or updates have been successfully completed, the compliance model number will not need to change. Therefore, it will not be necessary to contact each entity that has approved the product Compliance Model number.
Summary:
Grouping of product configurations into a single Compliance Model Number can save considerable time and money during the entire product lifecycle. By combining multiple configurations into a single regulatory model number, the manufacturer has to only homologate a single model with the numerous regulators around the world. This also optimizes the documentation and collateral required by the product management, marketing teams, distributors, and sales teams.
By having the regulatory compliance reports created in a way that allows some variation of the internal components in a system will allow the manufacturer to maintain the variations in the configurations without having to update the reports each time a new component is envisioned for the product. In some circumstances retesting will be necessary, but when updating the reports following the test program, ensure that there is flexibility in the list of possible components and there is provision for new generic alternatives.
Therefore, implementing a system of CMN strategy will simplify the product regulatory efforts and improve the time to market.