The Role and Responsibilities of a Product Regulatory Compliance Professional
By Tom Killam and Cyril Mecwan
June 01, 2021 | Series 1 / No. 4
The Product Regulatory Compliance professional is a warrior and an unsung hero, whose contributions are not overtly visible, not well understood, and hence not fully recognized. However, in order to ship products successfully to various target countries and markets, completed deliverables by the Compliance professional are required.
Background:
In the modern business history, a formal test lab opened in 1878 in Europe and in 1894 in the U.S. With the advent of technology and engineering, the testing function evolved into a multi-disciplinary approach. Today, advanced regulations are in place covering the testing of products in different industries and disciplines. In parallel, the role of a Product Regulatory Compliance professional has evolved to cover the comprehensive test requirements.
The Role and Responsibilities of a Product Regulatory Compliance Professional:
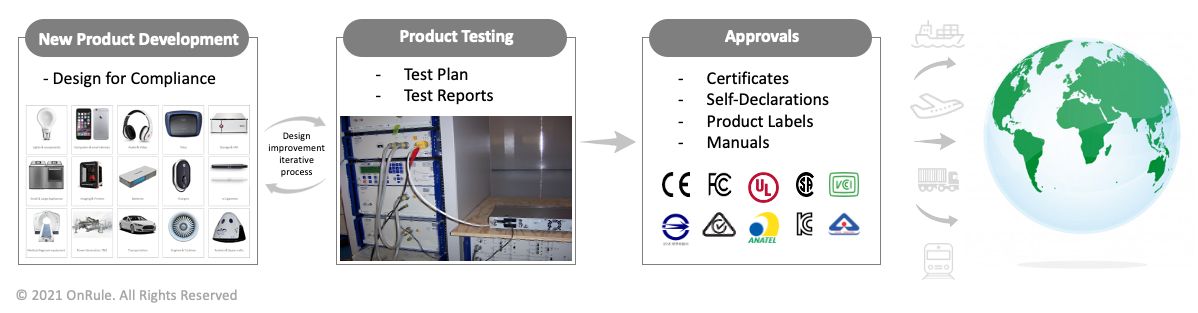
- Assessment of the Product Regulatory Compliance Landscape:
- New Product Introduction:
- The Compliance professional evaluates a new product for the specific technologies. In doing so, s/he classifies the technical category, class, and subclass of the product; intended environment in which the product will operate; critical components used; power level; and the inputs and outputs. This mapping lays the foundation of clarification for the overall compliance deliverables that must be met in order to get the compliance approvals from the regulators of the core countries and markets. Importantly, this understanding also defines the scope of work involved in getting the new product tested and approved.
- The Compliance professional communicates product technology of the new product to the test lab partners, invites quotes for testing, and works closely with the development manager and new product program manager in making the decision of selecting an appropriate test lab partner. In addition to the cost, the capability of the test lab in performing the tests specific to a discipline is considered. Also, the timeline of completion is another key determinant in choosing the test lab. Lastly, the test lab’s ability in providing the future support for the global market access is also considered. In consultation with the test lab partner, the compliance professional also advises the team of product marketing, program manager and development manager on the number of prototypes required for the purpose of testing.
- While the product is being designed, the Compliance professional works with the engineering team members to draft a test plan and test configuration. This upfront preparation of the testing collateral allows the testing team to start the testing process without any delay and as soon as the prototype arrives. In parallel, necessary regulatory markings are identified, and the product labels are being designed and approved. Moreover, the requirements for the Compliance Manuals are outlined and communicated to the Technical Publications teams so that the framework of the manual is created.
- Once the first prototypes arrive, the Compliance professional obtains the samples, sets up equipment at the test labs, and monitors the functionality of the Equipment/Unit Under Test (EUT/UUT). Most of the hands-on Compliance professionals spend significant time at the test lab during the time of testing. S/he communicates the results of the testing to the development organizations, identifies the design related weaknesses and deficiencies as supported by the test data, and requests corrections. As and if required, the design is revised to address the bugs; new prototypes manufactured based on the revised design are now regression tested to verify the bugs are addressed and ensure the design is more robust and will comply with the requirements.
- Once the final test reports, labels and manuals are available, the Compliance professional collaborates with the test lab and regulator to obtain the Type Approval Certificate or issue a Self-Declaration.
- Operations and Sustaining Phase:
- Global Market Access: Once the product is well established in the core markets, the business may decide to launch the products into multiple other countries with an aim to grow the market share. The Compliance professional works with the test lab partners, country-specific consultants, and country representatives or importers to apply and obtain the necessary regulatory approvals for the new markets. This may require applying to various regulators within a country and furnishing the core deliverables in support of the application. Some markets even require country-specific testing, whereas some markets may further ask for an in-country testing. The Compliance professional coordinates the overall efforts with the stakeholders and provides appropriate visibility of testing and approval status to the product managers and program managers.
- Expirations and Recertifications: The regulatory approvals, typically the Type Approval Certificates, for many markets are issued for a limited period which varies by country from one to five years. A few countries offer the Type Approval Certificates for the life of the product. It is the job of the Compliance professional to keep an eye on the upcoming expirations, so that the recertifications of the products will be completed prior to expiration. This in turn calls for proper budgeting and resource planning.
- A System of Record-Keeping:
- Audits
- Participation in Professional Organizations & Events:
- Standards Development Organizations (SDO): Senior Compliance professionals with years of experience and knowhow understand the overall standards landscape applicable to her/his field of expertise. They keep track of upcoming changes, and seek to understand the impact of these changes on their industry, business, and products. They inform the development teams so that the new products could be designed and tested with these upcoming changes in mind such that the products already in the market do not have to undergo retesting for the upcoming requirements. In some cases, the Compliance professional after years of hands-on experience may be invited to participate in the Technical Committees (TCs) that create and manage the standards.
- IEEE and Trade shows: Senior Compliance professionals participate in the local chapters of the IEEE, network with the local industry professionals through this, and exchange the ideas and industry updates. They also have an opportunity to publish articles with the IEEE. Importantly, they may attend the Trade shows organized for different disciplines such as EMC, Product Safety, Radio, Telecommunications etc. and for different industries, which offer them an opportunity to learn, present and network. These venues are source of excellent information; networking with the exhibitors such as the test lab partners provides them with an exposure to the latest test equipment and test methodologies, while keeping them abreast of various testing capabilities.
When a Compliance professional joins a new organization, s/he takes time to understand the company products – product type, product technology, configuration, components used; key active global markets; the distribution channels; and special or specific entities used such as a carrier. Then s/he becomes familiar with the current regulatory records to understand the disciplines involved, types of test reports generated, technical standards used, and test labs partnered for conformance testing.
To minimize the design iterations and testing cycles, to lower the overall costs of bringing a new product to the market, and to meet the time-to-market objectives, the new product introductions and development organizations in successful enterprises provide an upfront seat to a compliance professional on the new product introduction table. The involvement and opinion of a Compliance professional as early as the conceptual phase of a product brings beneficial contributions.
As the new product testing process is progressing, multiple documents are generated before, during and after the testing. An easy access and quick availability of compliance records - as a proof of approval for a market - is critically important. Salespeople want to make sure they can legally sell the product in a market; channel partners & distributors must have these records at their disposal before finalizing the contract with the OEM whose products they are selling; logistics & shipping personnel need the mandatory documents to be shipped along with the products; and auditors need them readily at the time of audit.
Therefore, record-keeping is an important function, which starts with organizing the compliance records for appropriate categories, disciplines, products, and markets. The Compliance professional creates a system of compliance records, organizes the documents, ensure they are securely stored and quickly available, and provide visibility of the records to the stakeholders as required.
Certain regulators, including but not limited to, UL in the USA and CCC in China require an audit of the factory that makes the end product seeking regulatory approval. The Compliance professional coordinates, prepares for and accompanies such an audit to ensure the OEM is properly represented. Similarly, regulatory audits are taking place at the company level OR shipment is rejected for the lack of regulatory approval – the Compliance professional is the main contact steering the audit and responses required against the queries. Additionally, some regulators require routine factory visits to perform an audit of the product being produced. These audits are to ensure that the product is identical to the product originally approved. These audits can occur up to four times a year.
Role of a Product Regulatory Compliance Consultant:
As an employee of a company, a Compliance professional is generally responsible for all the areas identified in the above section.
However, a start-up company often does not have a full appreciation of the compliance activities. Also, in the early stage, the company’s needs might not be big enough or it may not have the required budget. These possible reasons preclude the company from bringing a full-time Compliance professional onboard as a regular employee of the company. Instead, the company may opt to have a consulting arrangement.
A Compliance Consultant brings the experience of working on disparate technologies, has an expertise to resolve the compliance-specific issues in the product, and has a huge network of test labs and other country-specific consultants that allow her/him to yield quick results. The consulting engagement allows the company to quickly obtain the product regulatory compliance while keeping its cost variable.
We have observed that often the mid-size companies employ their compliance employees to manage the daily execution of compliance-specific projects and records, but invite the compliance consultants to solve the focused engineering problems for a new product, or manage a one-time project of organizing the overall compliance space, or facilitate/conduct an audit of company’s compliance processes to ensure the best practices are implemented in the company. We have also seen the Compliance consultants being brought in to support the testing activities performed using the captive or third-party test labs.
Lastly, a Compliance consultant is engaged to meet the objective of quickly but systematically launching a product globally. In the world of Product Regulatory Compliance, this activity is termed as ‘Global Market Access’. The consultant coordinates the overall efforts with test labs; the consultant also brings the regulatory know-how specific to a country.