Issues & Needs 5 - Given any country, what are my product regulatory compliance requirements?
By Tom Killam and Cyril Mecwan
September 14, 2021 | Series 1 / No. 9
One of the most common question we confront is: 'I want to launch a new product into twenty new countries. What product regulatory approvals do I need? What are my company’s deliverables?'
Initiated in IBM's early days (at least 50 years ago), the Product Safety Review Board (PSRB) has been conducting a product safety review for every new IBM product to ensure the safety of the product, its users and its environment when placed on the market. This was the era before the globalization, when the country-specific requirements for product regulatory compliance were a guarded knowledgebase, and very few professionals had the understanding and information of the global regulatory requirements. The big enterprises such as IBM, Siemens, HP, NCR, Northern Telecom (later it became Nortel Networks) etc. had painstakingly collected the regulatory requirements with a combination of forming relationship with the regulatory and customs bodies of the individual countries as well as working with local, in-country consultants that were familiar with the approval authority.
Although today, the awareness of product regulatory compliance is widespread, its knowhow is not yet ubiquitous or common knowledge, and due to its multi-dimensional nature, this space is still complex, which requires further education, implementation of best practices, simplification, and automation.
Background:
Acquiring product regulatory compliance for disparate markets requires fulfillment of many deliverables. Depending on the technology, the product is tested for product safety, EMC, Radio, Telecommunication, Energy Efficiency, RoHS, etc. The deliverables may include, but not limited to, test reports, type approval certificates, self-declarations, manuals, labels, markings, etc.
Issues:
The scope and extent of regulatory requirements for a product are determined by mapping the categorization of the product to a country’s regulatory requirements. Then the questions arise are: what is the categorization of the product? And what are the regulatory requirements for a country for the stated category of a product?
- Categorization of a product:
The answers to below questions help categorize a product.
Is the product categorized as an Information Technology Equipment or Medical Electronics or Appliance or Automotive? What is the Class of the product? Is there any Sub-class? What is the Intended Environment in which the product operates? What are the Technologies used in the product? What are the product Interfaces? What is the Power Source and Power Level?
Although answering the above questions is not difficult, developing a proper framework of hierarchy of categorization is critically important.
- Regulatory requirements:
Mapping the product categorization to the regulatory compliance requirements for a market determines the scope and extent of overall product regulatory requirements. However, gathering the country-specific requirements is a difficult part; maintaining the information is even more difficult as the requirements are continuously evolving and therefore are dynamic in nature. In the below representation and example, launching a new product in the USA - a Printer with Radio, Fax, and Laser capabilities - will require the OEM to complete 20-plus deliverables related to product regulatory compliance!
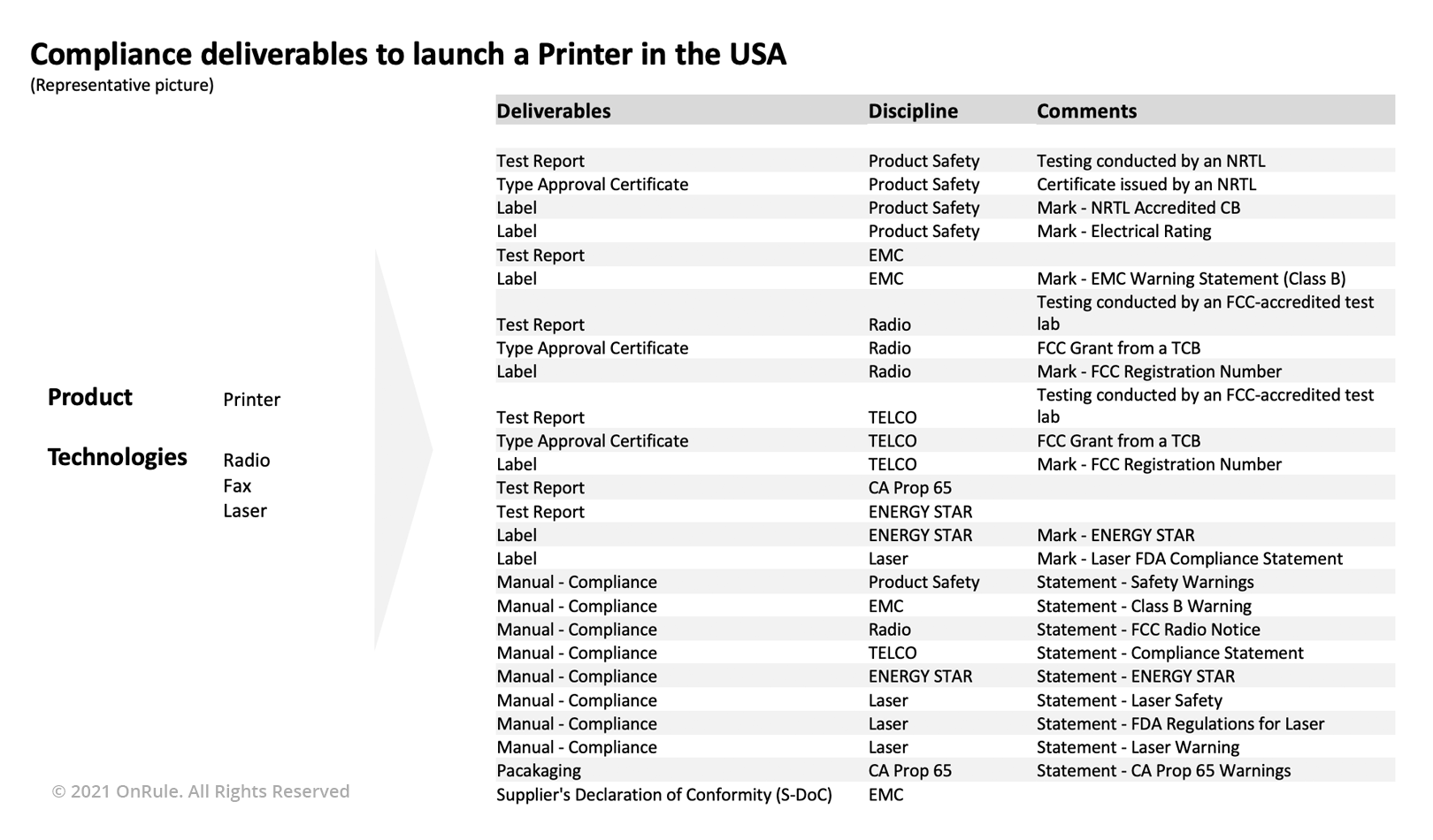
Once the product is approved for the core markets, the Product Manager would now like to further expand the market, and therefore obtain approvals for many more countries. Gathering the requirements for product regulatory compliance for big countries may be easier, but collecting the list of compliance requirements for smaller markets is relatively difficult. Additionally, language in which this information is available, and its interpretation may also increase the overall complexity in determining the end requirements related to product regulatory compliance. Although the test labs and Compliance consultants provide essential support to the Compliance professional during navigation through the Global Market Access, an incorrect or incomplete list of requirements adds to the churn, increases the cost of compliance and importantly, delays the approval. At the end of the day, the Compliance professional working for the OEM carries a big responsibility on her/his shoulder to ensure the timely acquisition of the product regulatory approvals.
Needs:
Therefore, having the automated intelligence for the product regulatory compliance at his/her disposal will strengthen the Compliance professional and augment his/her capabilities. A solution is required that guides a Compliance professional through a systematic query to determine the overall composition of the product including the type of product and technologies it utilizes, and then using built-in intelligence of requirements, propose the regulatory compliance deliverables for a given product-market mix. For a given product and technology, presenting to a Compliance professional a list of suggested compliance deliverables for 100 such countries will bring the required knowledge base and ease the complexity; importantly, it will greatly enhance the productivity and shorten the time to market. Today’s Compliance professional deserves automation and servicing in order to be more productive and efficient.